
Tailored Solutions for Your VNS Research Needs
We understand that every study brings unique ideas and requirements, which may call for customization. At Vagustim, we’re here to help. Whether it’s tailoring our vagus nerve stimulation device, ear electrodes, stimulation protocols, or other components, we’re committed to collaborating with you to meet your specific research needs. Feel free to reach out and share your ideas—we’re excited to work with you to bring your vision to life.
Versatile Solutions for Human and Animal VNS Research
Vagustim is designed to support both human and animal studies, making it a versatile tool for a wide range of research applications. Our specially developed ear clip electrodes enable non-invasive vagus nerve stimulation in animal studies, providing researchers with reliable and effective options for their unique study requirements.
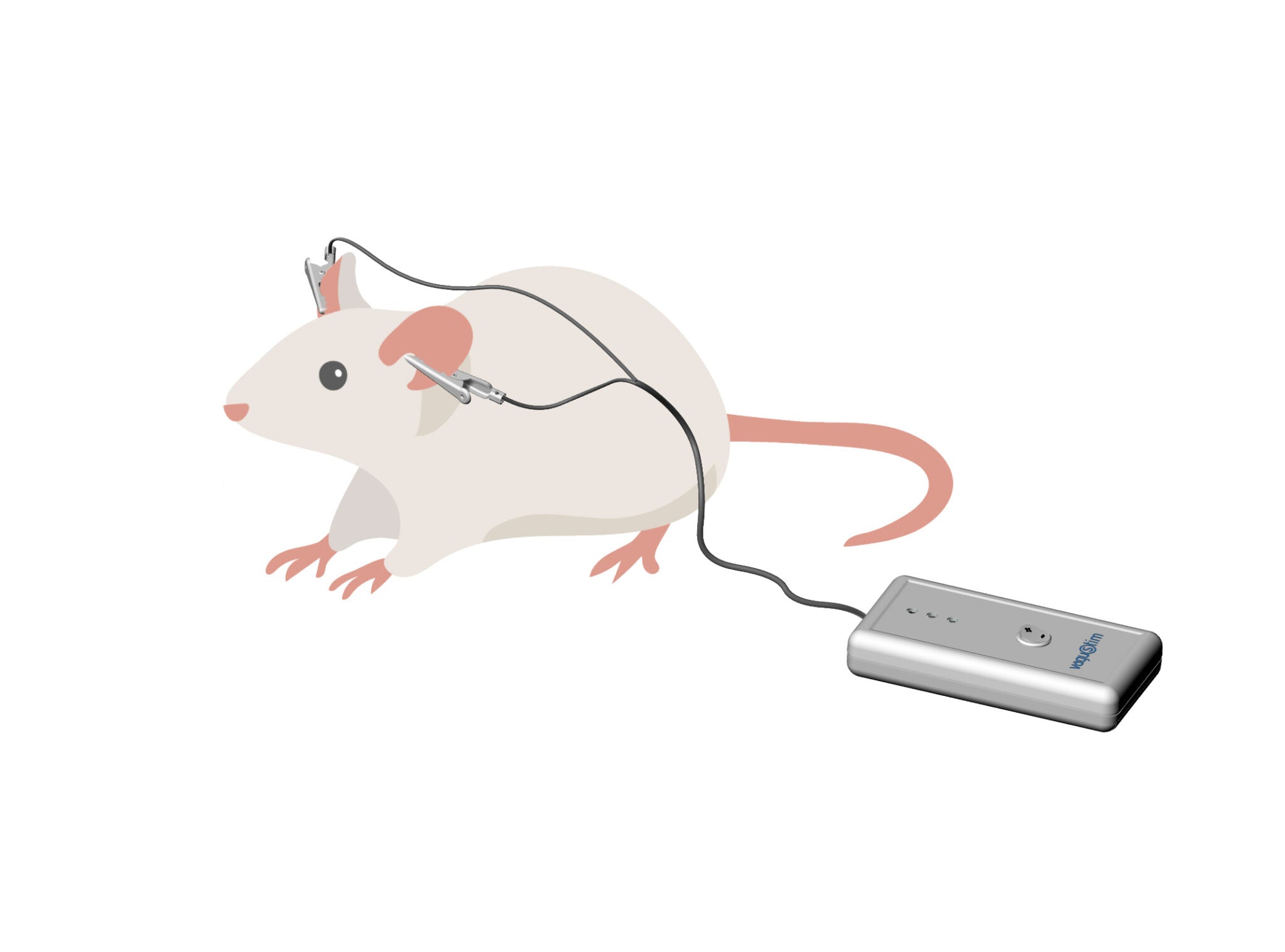
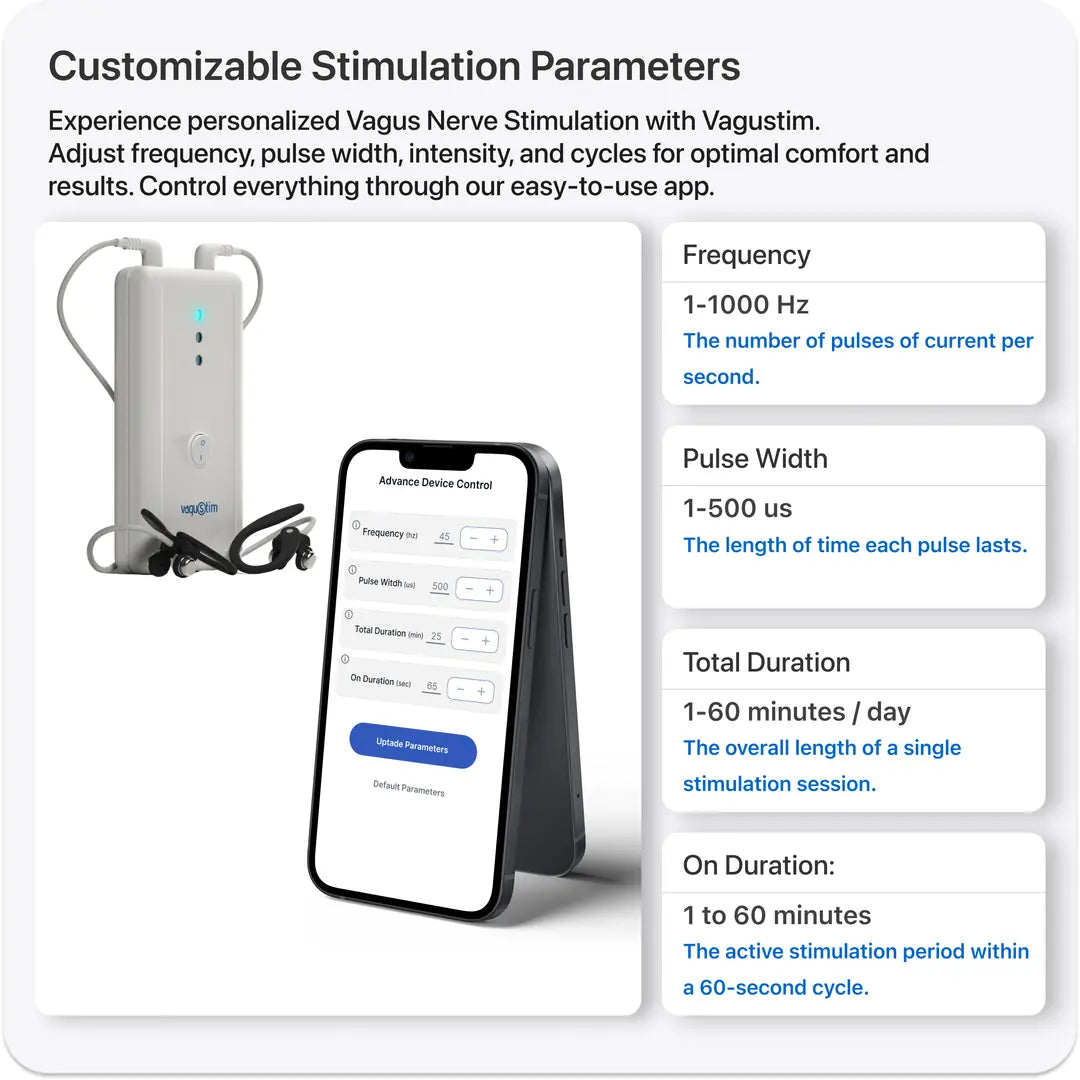
Advanced Features for Tailored Research
Vagustim offers a range of cutting-edge features designed to meet the needs of advanced studies. These include the option for bilateral or unilateral stimulation, current or voltage stabilization, various waveform options, versatile stimulation parameters, remote control capabilities, stimulation history logging, and the ability to apply customized pre- and post-session questionnaires. With these advanced tools and more, Vagustim is your ideal partner for tailored research solutions.
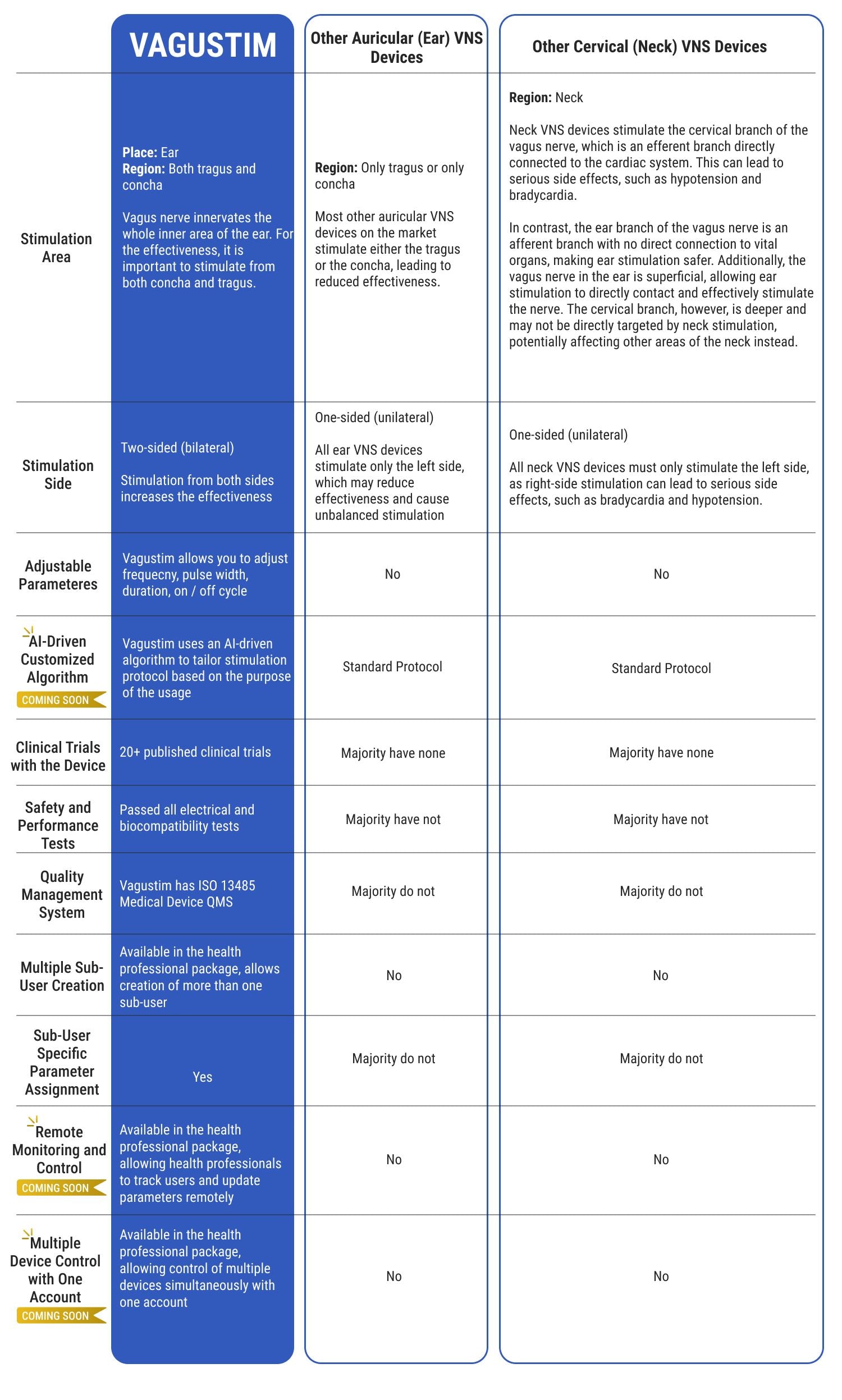
Why Vagustim?
Frequently Asked Questions
Please read our frequently asked questions to find out more
Can Vagustim be customized for specific studies?
Yes, Vagustim can be tailored to meet the unique requirements of your study. We offer customization options for the device, ear electrodes, stimulation protocols, and other parameters to align with your research objectives
Is Vagustim suitable for animal studies?
Absolutely. Vagustim features specialized ear clip electrodes, enabling non-invasive vagus nerve stimulation in animal studies, making it suitable for a wide range of research.
Do I need ethical approval to use Vagustim in research?
As a Non-Significant Risk (NSR) device, Vagustim typically requires ethical committee approval for research. We can provide necessary documentation to facilitate this process.
Is there technical support for researchers?
Yes, our team offers technical support, including setup assistance, training, and troubleshooting, to ensure smooth integration of Vagustim into your research workflow.
How can I get started with Vagustim for my research?
Contact us at sales@vagustim.io to discuss your study needs, request a quote, or learn more about how Vagustim can support your research goals.
Is Vagustim FDA Approved or CE Marked?
Vagustim is not FDA approved as it is currently categorized as a general wellness product under the FDA's General Wellness Guidelines. While not a medical device, it has been utilized in numerous studies and meets medical device standards. Non-invasive vagus nerve stimulation (VNS) is classified as a Non-Significant Risk (NSR) device, meaning ethical committee approval is sufficient to conduct research.
Vagustim does hold CE certification, but this certification is for general use and not a medical device CE mark. Despite this, its design and standards make it a reliable option for wellness and research applications.
Is my data safe with Vagustim?
Yes, your data is safe with Vagustim. We prioritize user privacy and data security, adhering to strict industry standards. All data collected by the Vagustim app is encrypted and stored securely. Users maintain control over their data, and it is never shared with third parties without explicit consent.
Additionally, Vagustim can be used without the mobile app, ensuring that no data is stored online.
Can I talk with researchers who have used Vagustim in their studies?
Yes, we are happy to connect you with researchers who have utilized Vagustim in their studies, whenever possible. Many researchers are open to sharing their experiences and insights about incorporating Vagustim into their work. If you’d like to be connected, please reach out to us at sales@vagustim.io, and we’ll facilitate introductions where appropriate, respecting privacy and consent.








